- Author:
- VCU STEM Project 2, VCU STEM Project 1, VCU STEM Project 3
- Subject:
- STEM/STEAM, Science, Matter
- Material Type:
- Lesson Plan
- Level:
- Middle School
- Grade:
- 8
- Provider:
- Virginia Commonwealth University (VCU)
- Tags:
- License:
- Creative Commons Attribution Non-Commercial
- Language:
- English
- Media Formats:
- Downloadable docs, Text/HTML, Video
Education Standards
Chemical Bonding Process Student Presentations directions storyboard Assignment Hand out
Chemical Bonds Compare & Contrast ALTERNATE option for online Sort Venn Diagram with Sort
Engage Chemiscope video notes v.2
Explanation of Chemical Bonds PS.3c v.2
Exploration PS.3 Substance Sort Chemical Bonds
Figure 3 Sample Resources
PS3c Lab Electrolysis of water
PS.3c PHET Investigation of Solutions of Ionic and Covalent Bonds
Video: Chemical Bonding Rap
Video: Chemiscope Catches Chemistry in the Act
Video: Directions for Chemical Bonds Compare and Contrast Activity
Video: Dogs Teaching Chemistry - Chemical Bonds (1)
Video: What is a Molecule?
Video: What is produced when the covalent bond in water is broken?
The Formation of Chemical Bonds
Overview
Students will explain that matter consists of atoms held together by electromagnetic forces and exists as different substances which can be utilized based on their properties. Students will be able to describe the behavior of atoms during a chemical change. Students will be able to distinguish covalent and ionic bonds.
This module was developed by Patricia Kramolisch as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.
Introduction
Overall goal of the module: Students will explain that matter consists of atoms held together by electromagnetic forces and exists as different substances which can be utilized based on their properties. Students will be able to describe the behavior of atoms during a chemical change. Students will be able to distinguish covalent and ionic bonds.
Target population: Physical Science students
Brief description of the lessons: The lessons within this module follow the pattern of a descriptive learning cycle. Teachers engage students’ attention to the submicroscopic scale of chemical bonding through discussion of prior experience with a light microscope. Then, student perspective is scaled down to the submicroscopic level with the anchor phenomenon video, “Chemiscope Catches Chemistry in Action”. Discussion of the video results in student groups generating a poster with their current understanding of compound formation and questions to investigate. Next, students are assigned roles to work in a cooperative learning group to research to find the answer to the consensus class essential question, “During a chemical change, how are the atoms behaving?” Students are provided with cards to sort in the categories of ionic bond, covalent bond, and element. By sorting sample substances and matching them with descriptive single element Bohr model cards of their components, students look for characteristic patterns of each category. This Substance Sort activity is documented by each group in a triple Venn diagram. The class completes a gallery walk of the Venn diagrams to compare their findings. Students revisit their initial questions and ideas they wrote after viewing and discussing the video to revise and add to their initial thoughts. Next students work in research groups to identify specific vocabulary and processes to accurately describe ionic and covalent bonding. The teacher interacts with groups to check for understanding and provide formative feedback. The teacher will interject direct instruction when needed to clarify misunderstandings. Optional lab station investigations are provided for teachers wishing to broaden students’ understanding of the influence of the type of chemical bond on the properties of a compound.
Objectives by lesson:
- To become chemically stable, the atoms of elements gain, lose, or share electrons (PS.3 c).
- Compounds can be classified as ionic or covalent based on the type of chemical bonds they contain (PS.3 c).
- When a metallic element reacts with a non-metallic element, their atoms gain and lose electrons respectively, forming ionic bonds. Generally, when two nonmetals react, atoms share electrons, forming covalent (molecular) bonds (PS.3 c).
Prerequisites:
- basic knowledge of atoms within matter
- atoms of different elements combine to form compounds
- the Bohr model of the atom
- the classification of elements into the categories of metals, metalloids, and nonmetals
- the chemical property of reactivity.
List of materials needed for the module:
- response papers for student work (Engage Chemiscope Video Notes, Substance Sort Cards with Triple Venn Diagram, Explanation of Chemical Bonds graphic organizer, Chemical Bonds Review with questions)
- video called, “Chemiscope Catches Chemistry in the Act” https://youtu.be/nhqAc0pk8i8
- internet access and/or print research sources
- poster paper
- Markers
- https://www.msichicago.org/play/goreact/ (internet based resource)
- Chemical Bonds Compare and Contrast PowerPoint
- Venn Diagram with Questions to Use with PowerPoint slide
- Chemical Bonding Process Student Presentation - Student Hand Out provides a storyboard for planning
- Chapter 4 Student Reading from Middle School Chemistry Unit by ACS
- Video: Chemical Bonding Rap (included with module)
- Video: Dogs Teach Chemistry (included with module)
- Lab materials for electrolysis of water: (Materials for each station) 9-volt battery, 2 wires with alligator clips on both ends, 2 pencils sharpened at both ends, water, spoon, epsom salt (magnesium sulfate), clear plastic cup, tape. The battery is placed between the upper halves of the pencils. Wrap tape around all three items to hold them together. Half fill the clear plastic cup with water. Add 2.5 g of salt to the water. Connect the top of each pencil to one terminal of the battery. Place the bottom ends of the pencils in the salt water. Safety tips: Instruct students to keep the battery and their fingers out of the water. Also, the battery will get hot. Students should keep watch on the bottom ends of the pencils in the water. They record observations of the bubbles that appear. Students should see a distinct difference in the amount of bubbles produced from each pencil. There will be twice as much bubbles from one side as compared to the other.
- Lab materials for conductivity of salt vs. sugar water: (Materials for each station) sugar, salt, three 500 mL beakers, water, conductivity tester, spoon. Label beaker and prepare beakers. One beaker with a salt water label is filled with a solution of 250 mL water and 5 g of salt. One beaker labeled as water is filled with 250 mL of pure water (distilled is best, however, tap water usually works fine). One beaker labeled sugar water is filled with a solution of 250 mL water and 5 g of sugar. Advise students about safety concerns with an electrical device. Students should not touch the plug with wet hands. Students should not touch the open circuit of the conductivity tester. Students should be directed to identify the control (pure water). Also, students are directed to rinse the conductivity tester after each use to prevent cross - contamination of samples.
- Lab materials for melting point lab: (Materials for each station) salt, sugar, chemical microspatula, metal spoon or evaporating dish or watch glass, bunsen burner or hot plate, safety goggles. These materials are used to compare the melting points of sugar and salt. If using a bunsen burner, place a single microspatula scoop of salt on the front of a metal spoon. Place a single microspatula scoop of sugar on the back of the same metal spoon. The metal spoon bottom is gently heated by the bunsen burner while moving the metal spoon slightly forward and back. Be aware the sugar melts quickly, will decompose, and may burst into flames. This all stays on the spoon. It is easy to manage by having the students prepared for what may happen and instructing them ahead of time to calmly put the spoon in the nearby sink (or other receptacle designated by the teacher), when done with their observations. Alternatively, the salt and sugar melting point comparison may be observed by melting the samples on a hot plate. Place one microspatula scoop of each on a watch glass. Turn off the hot plate as soon as the sugar melts. If a watch glass is not available, each sample may be placed in separate evaporating dishes. The teacher directly supervises students as they take turns with this activity.
Description of how objectives are assessed in the module: Continual formative feedback by teacher throughout the unit. Two options for summative assessment include a performance – based assessment and multiple – choice questions.
Module Instruction:
In this module the teacher provides students opportunities to imagine and describe matter at a submicroscopic scale. Students use models to search for patterns among atoms. The teacher allows time for student discussion and discovery before introducing formal chemical bonding vocabulary.
Engagement
To engage students in a submicroscopic view of matter, students watch a short video called, “Chemiscope Catches Chemistry in the Act”.
Introduce the video with students participating in a 1 – 2 – 4 cooperative learning discussion about a microscope. The teacher shows the students a microscope, either an actual microscope or a picture of one. Allow students thirty seconds to write down how they have used a microscope in the past or how they have seen one used in a movie or television show. Most students will have experience using a microscope in life science class. Next, students turn and talk for 30 seconds to one other student about their experience using a microscope. Stop the pair – share and direct student pairs to combine into groups of four. Tell students they have one minute to work with their group to compare their experiences and combine their ideas into one list of reasons people may use a microscope. When announcing time is up, the teacher then calls on each group to share their list and writes these ideas on the board for all students to see. The teacher is not commenting to correct ideas. This is a sharing time to make student thinking visible.
This whole – class discussion is to elicit students’ prior knowledge and to begin the process of bridging students’ thinking from the macroscopic to submicroscopic scale. The research of Kmel et al (1988) revealed the following “hierarchy of increasing cognitive demand” for students as they learn about chemistry.
- Disappearance
- Displacement
- Modification
- Transmutation
- Chemical reaction (interaction)
Consequently, it is worthwhile to take this time to prepare students for the video. Students need time to engage socially through discussion and sharing of ideas as they begin to view matter at a different scale.
Now the teacher tells the students they will watch a video about observing matter on a smaller scale than a microscope offers. Atoms are too small to be seen with a light microscope. Each atom measures from about 0.1 nanometer – 0.3 nanometer. An analogy to put this scale in perspective is to say one nanometer is to one meter as a marble is to the earth. Scientists study the chemical composition of matter and the behavior of atoms with electron microscopes. The video we will watch is produced by a team of scientists using their invention called a “chemiscope”. The teacher writes the two words, microscope and chemiscope, side by side on the board. Ask the students what part of these words looks the same. As students respond, underline the common root word, “scope”. Ask students to think about the different prefixes of each word. Direct students to talk with their group of four to predict what this new invention might do. After about one minute allow each group to share their prediction. Tell the students, “Now we will watch the video. We are going to write notes the second time we watch the video. For now, just watch the video and see how the scientists’ use of the chemiscope compares to your group’s prediction.”
After this first viewing of the video, the teacher gives students the video response guide. The teacher reads the questions on the video response guide to the students.
- How does the chemicscope view of chemical changes compare to watching a chemical change in a beaker?
- What do you think is meant by the final statement in the video? “You might say the chemiscope is an agent of change. And that these bonds may be shaken or stirred.”
- Examine the images from the video clip shown below. We are focusing on the compound as a system of atoms that move together as energy is flowing (in and/or out) through the system. The images show the interactions among the atoms as time moves on.
Tell students to listen for answers to these questions as they watch the video a second time. The teacher pauses the video each time content is stated that provides answers to either of the first two questions and allow students time to write their responses.
The third question is designed for students to begin to form their understanding of the unseen factors that make chemistry happen. After the video finishes, explain to students they are to work alone without talking for the next thirty seconds labeling the pictures on their video response guide. These are screenshots taken from the video. Read the directions with the students.
- We are focusing on the compound as a system of atoms that move together as energy is flowing (in and/or out) through the system. The images show the interactions among the atoms as time moves on.
- Describe, in your own words, with labels you write on the pictures the components (parts) of the system and how they interact.
Demonstrate how to label one thing on the picture in your own words to put the students at ease about describing and questioning what they see and think about the pictures. This is a time to allow students to describe what they think is happening. Allow student errors.
After thirty seconds, students work with their group of four to share their ideas and write questions they have about how compounds form. The question prompt in the guide will support student thinking to consider all aspects of the system: the atoms, interactions among the atoms, the compound, the function of energy, and how/why/what the model represents. This session of team discussion should be structured to involve all students. One possible way to do this is to give each student in the group two beans to represent their turns to talk. Students place their bean in the middle of the table as they take their turn. When all beans are in the middle, direct students to combine their descriptions of the pictures of the compound model of “chemistry in action” and their question lists on one large piece of poster paper. Encourage student groups to add additional questions they have formed as a result of their group’s discussion. Then, each group should hang up their poster in the classroom.
Exploration
The teacher will use the students’ ideas and questions from the groups’ posters to lead into the exploration activity called, “Substance Sort”. This activity allows students to take a closer look at the parts of compounds by matching examples of chemical compounds with atomic models of the elements in their formulas. To contrast the elements that form compounds with those that do not form compounds, two noble gases are included in the activity.
To introduce the Substance Sort activity, the teacher reminds students of their ideas and questions about how the atoms were separating and coming back together in the video. Some students may have made comments about the “stick” connecting the atoms in the model. The teacher uses these ideas to lead into a brief demonstration that highlights the role of energy in the formation of chemical bonds. As teachers we know free atoms bond together to attain a lower energy state as part of a compound. Energy is needed to break bonds and energy is released when bonds form. The goal of this demonstration is not to explicitly teach these facts but to bring context to the questions students have expressed. Students have an opportunity to visualize this flow of energy within a system during this demonstration. Give each student a rubber band. Direct students to hold the rubber band between their two hands. In this experience we are thinking of each hand as an atom and the rubber band represents what holds them together. Tell students to stretch the rubber band by pulling their hands apart and then to bring their hands back together. Direct students to talk with their partner about the energy they used and which movement was easier.
Use this context to bring students to the essential question, “During a chemical change, how are the atoms behaving?” To explore this question students will work in cooperative learning groups. It is best to structure this activity with student roles that define their responsibility. The teacher should have established norms for students to practice active listening skills to let each student speak without judgment from peers. One sample for student roles from the Tools for Ambitious Science Teaching and modified conversation prompts are listed below. It is important to model and practice use of these prompts before the students use them. It is also important to provide a copy of the norms, roles and prompts in print to the students while they are expected to use them during group work. Some teachers put them on table tents. Others provide these norms, roles and prompts on a bookmark or on the students’ paper.
Big ideas person: Focuses the group on the scientific purpose of the activity.
- Asks “What pattern do we see with X (the atoms, the valence electrons, the type of elements, the compound) in this substance?”
- Asks: “How does X change the way we’re thinking about the chemical change?”
- Asks: “What is the big idea we are trying to understand? Why are we examining the atomic models?”
Clarifier: Monitors group members’ understanding of key science terms.
- Asks: “Do we know what the word bond refers to?”
- Asks: “Can we put it into our own words?”
- Asks: “How might the atoms be interacting?”
- Asks: “Why does our model have some elements as single atoms?”
Questioner: Keeps the conversation going within the group by paraphrasing what others have said, asking group members to build on each other’s ideas, and asking new questions.
Paraphrases what other have said: “So, what I think you are saying is… Is that right?”
- Asks: “What does it mean that ____?”
- Asks: “How do we know that_____?”
- Asks: “What is it important that______ ?”
- Asks: “What’s your evidence?”
Progress monitor: This person asks others to periodically take the measure of the group’s progress.
- Asks: “What can we say we’ve accomplished so far?”
- Asks: “What do we still need to know/do to accomplish this task?”
- Asks: “What can we now add to our explanation that we didn’t have before?”
To proceed with the Substance Sort activity the teacher gives each group of students a triple Venn diagram template and sets of cards with information the students will sort, analyze and discuss. The steps for conducting the activity are announced to the students as they proceed. A sample script for the teacher follows.
- During this activity you will look at examples of the products of chemical changes. Record your observations in the triple Venn diagram. Observations that are unique to a category are written in the single circle. Ideas and facts that are common to more than one category are written in the section of the Venn diagram that overlap.
- You are given three categories. Find these category name-cards: ionic compounds, covalent compounds, and elements. Place these in one row from left to right across your table.
- Next, I am giving you sample substances to sort into these categories. (Give students the card set that does not have the pictures of the Bohr model of atoms.) The “Progress Monitor” in your group deals the cards to your group members until all cards are evenly distributed.
- Take turns going around your circle allowing each member to read one of their cards and place it in the matching category. As you place your cards compare them to others in the same category and look for patterns. Remember to play your role to question each other and identify ideas your group should write in the triple Venn diagram. Continue going around the circle until all cards have been read and categorized.
- The last set of cards you are receiving are the individual elements with a picture of its Bohr model and descriptive facts. The “Progress Monitor” deals the cards to your group members until all cards are evenly distributed.
- During this part of the activity it is especially important for the Big Idea Person to focus the group on the purpose of this activity. Remember our essential question, “During a chemical change, how are the atoms behaving?” So, look with me at examples from the questions on the Big Idea Person’s list which may keep the group discussion moving to figure this out.
- “What pattern do we see with the atoms in this substance?”
- “What pattern do we see with the valence electrons in this substance?”
- “How pattern do we see with the types of elements in this substance?”
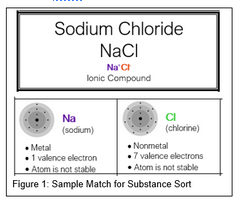
- Let’s discuss one compound together as a class. In your Ionic Compounds category find sodium chloride, NaCl. Place the cards for sodium, Na, and chlorine, Cl, right under the compound card. (See Figure 1) You may not be able to answer the questions we just highlighted from the Big Idea Person’s list right away.
- The Questioner might ask, “What does it mean that the sodium atom has one valence electron?” or “Why is it important that sodium has one valence electron?”
- Continue working with your group. I will be moving among your groups to hear your discussions and see how you are making notes in your triple Venn diagram. You have 20 minutes to complete your Venn diagram. Then, you will have a chance to tour among the other groups’ and comment on their Venn diagrams (often called a gallery walk).
- As the teacher moves among the groups, she/he listens for misconceptions or student frustration. It is recommended to pause the class after approximately 10 minutes of work time and show one or two short video clips for students to compare with their thinking and their group’s discussion. The two video clips included with this module are “Chemical Bonding Rap” and “Dogs Teach Chemistry”.
The teacher moves among the groups to prompt students as needed and interact with each group. After approximately 20 minutes tell students to leave their Venn diagram in the center of their table. The students will participate in a gallery walk to view and comment on the Venn diagrams of other groups. It is recommended for the teacher to use a timer and announce to students to move to a new table every 3 – 4 minutes. Students should leave comments on the table for the group to consider in relation to their Venn diagram. When students return to their group, the Progress Monitor should read the comments left by the other students. Each student ends this time by returning to her/his personal notes on the video response guide. Students add to their questions and observations about how atoms are behaving during a chemical change. It is expected for students to have seen patterns but not know the reasons or functions of the various factors they described in their Venn diagrams. This exploration is intended to build student curiosity and motivation.
Chemical Bonds Compare and Contrast PowerPoint
The Chemical Bonds Compare and Contrast PowerPoint is a variation of the “Substance Sort” just described in this lesson. It is designed to support student learning by reducing the number of options a student will see at one time. This format reduces the cognitive load. This may be used in an online learning management system to provide an asynchronous learning experience.
- At the beginning of the PowerPoint is a direction to “Play the video for Directions for the Chemical Bonds Compare and Contrast Sort”. This video provides a “Think Aloud” example to model for the students the reasoning and justification for choices when sorting the elements to their matching compounds. This is a separate resource included with this instructional module.
- Each slide of the PowerPoint activity limits the students’ view to one example of an ionic compound, a covalent compound, and a noble gas.
- The activity could be adjusted for students learning English and students with disabilities by reducing the number of examples that the student should match and analyze.
- The teacher should model how to complete all steps of the example started in the direction video and write descriptors on the Venn diagram. Allow the student to keep a copy of the example.
- The teacher would assign the student one slide of the PowerPoint to complete using a Venn diagram with question prompts for what to describe in each circle. At this time, answer the questions for each category based on their observations of the atomic models provided in the PowerPoint slide.
- If possible, allow students to complete the activity with a partner.
- After students have done their own example, they compare their Venn diagrams with other students’ and work together to fill in the overlapping sections and describe what the different categories have in common.
Explanation
The students are now ready to find an explanation for these observations and questions they developed while investigating how atoms are behaving during a chemical change. The teacher provides students with several resources and a graphic organizer (see Figure 2 below) to complete. Each student completes a personal copy of the graphic organizer. However, students should divide the research questions among their team members, research, and then hold a group meeting to share their answers to their part of the research questions. The teacher may provide support to students by having students work as partners within their group. Or, students from different groups working on the same research question could work together. Then, these students will return to their original group for the team meeting to share their findings. The teacher will monitor student work. If the majority of the class seems to be stuck on a particular part of the research, the teacher will provide direct instruction to clarify misunderstandings.
As students are conducting research the teacher may provide video clips, Nearpod, interactive simulations and other resources. A sample list is shown in Figure 3.
It is recommended all students read a text source and use the interactive chemical compound forming game found at https://www.msichicago.org/play/goreact/ (website for the Chicago Museum of Science and Industry). Students select the featured reactions tab on the left of the website page to find common examples of compounds.Some of the descriptors with the compounds forming will refer to bond type. An effective eight minute video found on youtube called, “What is a Molecule?” Go to What is a Molecule video by clicking here.
The teacher dialogues with groups to make sure they are finding the connection between the patterns they discovered during the Substance Sort activity and the octet rule. Atoms with a full outer energy level, often referred to as a noble gas configuration, are in a lower energy state and chemically stable. For hydrogen (H) and helium (He) the ideal number is two valence electrons to form a full outer energy level. These are unique because they only have one energy level. For Physical Science we focus on the basic principle of this concept and do not include transition elements. The ideal number of valence electrons for elements to have a full outer energy level is eight. This is referred to as the “octet rule”. For example, students identified one atom of neon has eight valence electrons during the Substance Sort activity. The neon atom has a full outer energy level and is stable. Calcium has two valence electrons and is unstable (not stable). Students should annotate their own copy of the Chapter 4 Reading (Middle School Chemistry Unit, American Chemical Society, https://www.middleschoolchemistry.com/lessonplans/chapter4 ) selection provided in the resource section of this module. In particular, pages 354 - 361 of the Chapter 4 Reading provides students with the formal text and illustrated examples of the concepts they were discovering by completing the Substance Sort activity.
A summary of information students should gain an understanding of during the Explanation phase is described here.
Ionic bonds occur when a metal atom lends (gives) a valence electron to a nonmetal atom. This electron transfer changes the number of electrons on each atom. Now, the atom with unequal numbers of electrons and protons is called an ion.
- Metal atoms become positive ions when they lend (give) valence electrons.
- Nonmetal atoms become negative ions when they take (gain) valence electrons.
- The attraction of these opposite charges of the ions holds together an ionic compound.
- Example of a chemical change to form an ionic bond:
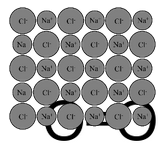
- The image shows a crystal lattice structure within sodium chloride (NaCl), commonly called salt. This alternating pattern of positive and negative ions holds together ionic compounds.
Covalent (molecular) bonds occur when nonmetal atoms vie (compete eagerly) for each other’s valence electrons. Each nonmetal atom has a nearly full outer energy level and will not give away electrons. This attractive force causes the atoms to share valence electrons and form a covalent bond. Also called a molecular bond. A common example is water. The image shows different models used to represent a molecule of water.
Within the water molecule, the hydrogen and oxygen have a “tug – of – war” for the valence electrons. The greater number of protons in the nucleus of the oxygen atom causes the oxygen side of the molecule to be slightly negative. The hydrogen side of the molecule is slightly positive. This unequal sharing of valence electrons makes bonds within the molecule polar covalent bonds. And, the water molecule is a polar molecule. An understanding of the polar nature of the bond within water supports the students understanding of the unique properties of water which they studied during the science in grade six.
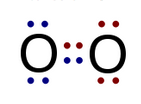
In a molecule with equal sharing of valence electrons the bonds are nonpolar covalent bonds. An example is two atoms of oxygen forming an oxygen molecule. The model on the left represents the valence electrons as dots. The model on the right uses one line to represent two shared valence electrons. Therefore, the two lines between the chemical symbols represent two covalent bonds.
Elaboration Phase of Instructional Module
To broaden students’ understanding of the influence of chemical bonds on the properties of compounds, the teacher would set up lab stations such as the following. The teacher may choose to set up each activity at two different locations in the classroom. This will allow for two sets of lab groups to rotate among five stations. Half of the lab groups will work through stations on one side of the room. The other half of the lab groups will rotate through lab stations on the other side of the room.
Elaboration
Lab Station Investigations
- Electrolysis of Water – Problem: What is produced when the covalent bond in water is broken?
- Conductivity of Salt Water vs. Sugar Water – Students use a conductivity tester to compare salt water to sugar water.
- PHET Investigation of the Conductivity of Salt Water and Sugar Water – Students use a virtual lab to compare the properties of salt (ionic compound) to sugar (covalent compound).
- Melting Point of Salt vs. Melting Point of Sugar – Students observe both compounds on a watch glass on a hot plate to compare their melting points. This may also be demonstrated by the teacher by placing samples of salt and sugar one metal spoon. Then, warm the bottom of the spoon by a Bunsen burner. The sugar melts quickly and, then, decomposes.
- Demonstrate or watch a video of the effect of a charged object on a stream of water coming out of a sink faucet.
Evaluation
Students may use information gained by completion of all activities in this lesson to create a presentation of chemical bonding in a format of their choice. A sample description of this type of a “Chemical Bonding Presentation” is shown below. This serves as a performance – based assessment.
The student hand out called, “Chemical Bonding Process Student Presentation”, has directions to guide students as they plan their presentation. Students will complete a storyboard to represent how they will make their presentation. This gives the teacher the opportunity to provide formative feedback and guide students with focused questions to think about their ideas, reteach, and correct misconceptions.
Assessment
Students may demonstrate their understanding with a performance-based assessment by presenting illustrated examples of each type of chemical bond in a booklet, video, Flipgrid or other format of their choice. The documents provided with this module include a sample assignment planning guide for this type of assessment.
Covalent bonds occur within molecules containing
Both metal atoms and nonmetal atoms
Only metal atoms
Only nonmetal atoms
Only metalloid atoms
Ionic bonds occur within compounds containing
Both metal atoms and nonmetal atoms
Only metal atoms
Only nonmetal atoms
Only metalloid atoms
The element sodium (Na) forms a compound with the element chlorine (Cl) by
sharing its valence electron
sharing all its electrons with chlorine
transferring its valence electron to the chlorine atom
taking valence electrons from the chlorine atom
Which of the following statements best describes the bond that occurs to form KF (potassium fluoride)?
Fluorine and potassium share their valence electrons.
Fluorine gives its valence electron to potassium.
Fluorine takes a valence electron from potassium.
Fluorine has a full outer energy level and will not bond.
Which of the following statements best describes the bond that occurs to form CaKr (calcium kryptonide)?
Krypton and calcium share their valence electrons.
Krypton gives its valence electron to calcium.
Krypton takes a valence electron from calcium.
Krypton has a full outer energy level and will not bond.
Resources
Tool Systems to Support Progress toward Expert-Like Teaching by Early Career Science Educators2008-2013 Discovery K-12 GrantNational Science Foundation No. DRL-0822016. https://ambitiousscienceteaching.org/tools-scaffolding/
AP Central - Ending Misconceptions About the Energy of Chemical Bonds http://apcentral.collegeboard.com/apc/members/courses/teachers_corner/49039.html 6/26/2008
Walton, Marsha. Chemiscope Catches Chemistry in the Act – Science Nation, https://youtu.be/nhqAc0pk8i8 March 13, 2014.
For more information about Virginia Commonwealth University's School of Education STEM initiatives, visit the Center for Innovation in STEM Education (CISTEME).