
See computer science integration opportunities in middle school science in this crosswalk of the two disciplines.
- Subject:
- Computer Science
- Science
- Material Type:
- Teaching/Learning Strategy
- Author:
- CodeVA Curriculum
- Date Added:
- 03/28/2023

See computer science integration opportunities in middle school science in this crosswalk of the two disciplines.
In this experiment, two chemicals that can be found around the house will be mixed within a plastic baggie, and several chemical changes will be observed.
This module is designed to guide students in better understanding light. The students will also understand how light travels and interacts with other materials. The teacher will facilitate students' explorations as they generate a summary of their experiences. Throughout this unit, students will be guided in using practical materials such everyday items found in their classroom and light energy produced by flashlightThe goal of this module is for students to explore light and to better understand how it behaves. This module has been designed for 5th grade students or students who are developmentally ready to explore light. This module could also be used as a review for students in upper grades who need to build their fundamental understanding.
Bridges come in a wide variety of sizes, shapes, and lengths and are found all over the world. It is important that bridges are strong so they are safe to cross. Design and build a your own model bridge. Test your bridge for strength using a force sensor that measures how hard you pull on your bridge. By observing a graph of the force, determine the amount of force needed to make your bridge collapse.
A bungee jump involves jumping from a tall structure while connected to a large elastic cord. Design a bungee jump that is "safe" for a hard-boiled egg. Create a safety egg harness and connect it to a rubber band, which is your the "bungee cord." Finally, attach your bungee cord to a force sensor to measures the forces that push or pull your egg.
A zip line is a way to glide from one point to another while hanging from a cable. Design and create a zip line that is safe for a hard-boiled egg. After designing a safety egg harness, connect the harness to fishing line or wire connected between two chairs of different heights using a paper clip. Learn to improve your zip line based on data. Attach a motion sensor at the bottom of your zip line and display a graph to show how smooth a ride your egg had!

There are two types of catalysis reactions: homogeneous and heterogeneous. In a homogeneous reaction, the catalyst is in the same phase as the reactants. In a heterogeneous reaction, the catalyst is in a different phase from the reactants. This activity addresses homogeneous catalysis.
In this interactive activity, learners build computer models of atoms by adding or removing electrons, protons, and neutrons. It presents the orbital model of an atom: a nucleus consisting of protons and neutrons with electrons surrounding it in regions of high probability called orbitals. Guided tasks are provided, such as constructing a lithium atom and a carbon-12 atom in the fewest possible steps. The activity concludes with a model for building a charged hydrogen atom (an ion). Within each task, students take snapshots of their work product and answer probative questions. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology.
Elementary grade students investigate heat transfer in this activity to design and build a solar oven, then test its effectiveness using a temperature sensor. It blends the hands-on activity with digital graphing tools that allow kids to easily plot and share their data. Included in the package are illustrated procedures and extension activities. Note Requirements: This lesson requires a "VernierGo" temperature sensing device, available for ~ $40. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology. The Consortium develops digital learning innovations for science, mathematics, and engineering.
In this physical science lab, students investigate whether or not chewing gum should be considered eating. Students plan their own experiments for this lab. They use the law of conservation of mass to reason that the portion lost of the original mass of gum must be swallowed. Students determine the portion of original mass of gum. A student lab sheet and CER template are provided.

The lessons in this module are empirical – abductive. The teacher helps students identify the activity of substances within pizza dough. The teacher announces the students will conduct chemical reactions to explore how matter is conserved during a chemical change. After the class compares their reasoning, the teacher provides clarifying and direct instruction with videos, guided practice and supported computer simulation practice for students to learn to balance chemical equations. Students complete a problem-based investigation to apply their learning by writing, testing and explaining a lab procedure that will help an absent classmate to gather evidence and gain an understanding of the Law of Conservation of Matter. This module was developed by Patricia Kramolisch as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.
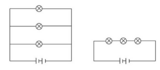
By the end of this module, the students will be able to explain (using physical models and computer simulations) the components of electrical circuits, the purpose of each component, and the differences between series and parallel circuits.This module was developed by Christina Owens as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.
Explore the concept of evaporative cooling through a hands-on experiment. Use a wet cloth and fan to model an air-conditioner and use temperature and relative humidity sensors to collect data. Then digitally plot the data using graphs in the activity. In an optional extension, make your own modifications to improve the cooler's efficiency.
As technology has evolved over time so has the understanding of the structure of the atom. This module focuses on how the model of the atom has changed over time using The Atomic Theory Timeline including the historical contributions of the scientists involved. This module was developed by Tracey Nipper as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.
Students will explain that matter consists of atoms held together by electromagnetic forces and exists as different substances which can be utilized based on their properties. Students will be able to describe the behavior of atoms during a chemical change. Students will be able to distinguish covalent and ionic bonds.This module was developed by Patricia Kramolisch as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.

Build your own miniature "greenhouse" out of a plastic container and plastic wrap, and fill it with different things such as dirt and sand to observe the effect this has on temperature. Monitor the temperature using temperature probes and digitally plot the data on the graphs provided in the activity.
Explore how the Earth's atmosphere affects the energy balance between incoming and outgoing radiation. Using an interactive model, adjust realistic parameters such as how many clouds are present or how much carbon dioxide is in the air, and watch how these factors affect the global temperature.
Make your own miniature greenhouse and measure the light levels at different "times of day"--modeled by changing the angle of a lamp on the greenhouse--using a light sensor. Next, investigate the temperature in your greenhouse with and without a cover. Learn how a greenhouse works and how you can regulate the temperature in your model greenhouse.

The student will be able to plan, conduct and analyze an investigation of thermal energy transfer.The student will be able to explain the three processes of heat transfer.The student will use science and engineering practices to investigate and clarify concepts of heat and thermal energy.This module was developed by Patricia Kramolisch as part of a Virginia Commonwealth Univeristy STEM initiative sponsored by the Virginia Department of Education.

Discover how electricity can be converted into other forms of energy such as light and heat. Connect resistors and holiday light bulbs to simple circuits and monitor the temperature over time. Investigate the differences in temperature between the circuit with the resistor and the circuit using the bulb.