- Author:
- VCU STEM Project 3, VCU STEM Project 1, VCU STEM Project 2
- Subject:
- STEM/STEAM, Science
- Material Type:
- Lesson Plan
- Level:
- High School
- Provider:
- Virginia Commonwealth University (VCU)
- Tags:
- License:
- Creative Commons Attribution Non-Commercial
- Language:
- English
- Media Formats:
- Text/HTML, Video
Education Standards
Cards for Modeling of Chemical Change in Pizza Yeast Dough
Chemical Reactions Module Assessment
Engage with Chemical Reactions Pizza Dough with Student Model Activity Copy with Notes
Explanation Law of Conservation of Matter
Explanation Law of Conservation of Matter Answer Key
Exploration Lab Law of Conservation of Matter Chem Reactions
Library For Self Documentation Science Template Chem Reactions
Conservation of Matter During a Chemical Change
Overview
The lessons in this module are empirical – abductive. The teacher helps students identify the activity of substances within pizza dough. The teacher announces the students will conduct chemical reactions to explore how matter is conserved during a chemical change. After the class compares their reasoning, the teacher provides clarifying and direct instruction with videos, guided practice and supported computer simulation practice for students to learn to balance chemical equations. Students complete a problem-based investigation to apply their learning by writing, testing and explaining a lab procedure that will help an absent classmate to gather evidence and gain an understanding of the Law of Conservation of Matter. This module was developed by Patricia Kramolisch as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.
Introduction
Goals
- The student will be able to describe how matter is conserved during a chemical change.
- The student will be able to model and explain how to balance a chemical equation.
Target Population Physical Science Students (8th grade)
Description The lessons in this module are empirical – abductive. The teacher helps students identify the activity of substances within pizza dough. The teacher announces the students will conduct chemical reactions to explore how matter is conserved during a chemical change. After the class compares their reasoning, the teacher provides clarifying and direct instruction with videos, guided practice and supported computer simulation practice for students to learn to balance chemical equations. Students complete a problem-based investigation to apply their learning by writing, testing and explaining a lab procedure that will help an absent classmate to gather evidence and gain an understanding of the Law of Conservation of Matter.
Objectives
- A chemical equation represents the changes that take place in a chemical reaction. The chemical formulas of the reactants are written on the left, an arrow indicates a change to new substances, and the chemical formulas of the products are written on the right (PS.3 d).
- The law of conservation of matter (mass) states that regardless of how substances within a closed system are changed, the total mass remains the same (PS.3 d).
Prerequisites Students should have an understanding of atoms, molecules, elements, compounds, and chemical changes.
Materials
- Activity cards for role play during pizza dough modeling activity
- Pizza Video PowerPoint
- Video: Demo - Conservation of Matter (included with module)
- Video: Alka Seltzer and Water in a Closed System (included with module)
- Video: Healthy Eating - Cooking Across Generations (included with module)
- Video: The Chemistry of Onions (included with module)
- Student hand out: Library for Self-Documentation Template Chem Reactions
- Lab materials
- graduated cylinders
- beakers
- lead (II) nitrate
- sodium iodide
- hydrochloric acid
- bromothymol blue
- Alka Seltzer tablets
- plastic bags
- rubber bands
- balances
- water
- chemical safety goggles
- weighing paper
- Copies of student hand-outs: Exploration Lab Investigation of the Law of Conservation of Matter, Guided Practice for Balancing Chemical Equations
- Modeling dough or mini geometric foam shapes
- Containers to organize lab and modeling supplies
- Internet access
- Final problem – based activity
- vinegar
- baking soda
- graduated cylinders
- plastic bags
- beakers
- rubber bands
- balances
- paper towels
- chemical safety goggles
- chemical spatulas or plastic spoons
Engagement
The teacher engages students’ attention to the concept of the conservation of matter during a chemical reaction with a video about pizza. Introduce the chemical reactions module with the American Chemical Society’s Reactions series video, “Why is Pizza So Good?”
The video will be watched two times. For the first viewing, tell students to count the number of different chemical changes mentioned in the video. A typical answer is four.
1. Dough rising
2. Baking soda to neutralize sauce
3. Cheese making
4. Baking pizza: Maillard reaction
Tell students prior to the second viewing of the video to pay special attention to the chemistry within the pizza dough. Watch only the first minute of the video. Ask students to consider the question, “How is matter conserved in pizza dough?” At this time, students will not have an understanding of the law of conservation of matter in the context of a chemical reaction. This is the anchor phenomenon to drive students to question and try to make sense of the idea of conservation of matter during a chemical reaction. This sets up the students to role play the chemistry of the pizza dough with the modeling activity described next. This module has an accompanying PowerPoint presentation (attached) to assist the teacher in guiding the class through this lesson. The modeling activity is not a balanced chemical reaction. The teacher is not to use this activity to teach vocabulary nor the concept of the law of conservation of matter. Its purpose is to help students visualize the chemical change and cause students to form questions that may be answered with the investigative activities in this module. During and after the activity the student will describe the ideas they are forming in their own words.
Students' Model of Chemical Change in Pizza Dough
1. Prior to class, the teacher prepares cards for the students to hold that identify each student’s role during the activity.
Print 2 copies of cards with images. Cut cards apart on the solid lines. Then, fold each card in half.
Print 1 copy of the SUGAR cards. Cut these apart on the solid lines
2. During class, assign student roles and give the students their cards. Tell students to hold the picture labeled as the “first substance” facing out toward the class.
10 students represent flour – give each of these students one flour image card and one sugar card (this is held behind the flour image card out of sight until it is needed later during this activity)
3 students represent water – give each of these students one water image card
2 students represent yeast – give each of these students one yeast image card and one container of bubbles with a wand for blowing bubbles
3. Direct students to gather in one area of the classroom. Students in the model walk around slowly within the space identified by the teacher.
4. The teacher announces the substances are gaining heat energy. The students walk faster and flip their cards.
5. As flour students flip their cards, they give the yeast students their smaller card labeled as sugar.
6. Then, the yeast students blow bubbles.
7. When students in the model see bubbles, they step apart and leave spaces.
8. The yeast students stop blowing bubbles, flip their cards and stand in the spaces.
9. The student model now represents the conclusion of the chemical change. Discuss with students the comparison of the student model with the image of the electron micrograph of yeast dough in the PowerPoint presentation (Figure 1).
10. Students use their observations of the student modeling activity to write a reflective summary of what they think the answer to the question, “How is matter conserved in pizza dough?”. Tell students they will further investigate the concept of the conservation of matter in the next lesson. Now students are “dumping” their thoughts as they write their reflection to begin to form their own definition of the conservation of matter in a chemical reaction. Students will still have questions and uncertainties. This is an opportunity for students to express their thinking.
Exploration
This lesson provides an exploration of the conservation of matter at the macroscopic level. And then, students will model the lab work at the submicroscopic level. This unusual routine is recommended to help students transfer what they are understanding with the lab experience to a visualization of how we make a model to represent the chemical change at the nano scale. Students often enjoy a lab on one day yet fail to connect it with the equations they will balance in the future. The intention of this activity is to combine the experiences and assist students in connecting the concepts. This will take more than one class period for students to complete all three stations.
If time is an issue, the teacher could have each lab group complete one station. The teacher provides a central location in the classroom for lab groups to share their data on class data record. Once each lab group has completed their work, the teacher would have a whole class discussion with groups sharing their experiences and findings.
To introduce the lab the teacher may use Harvard’s Project Zero Thinking Routine called, “I Used to Think, Now I Think”. Say to the students they will now take a few minutes to think about how their ideas about chemical changes has shifted. Use the following prompts to direct students to write down their thoughts. Then, allow time for students to share their responses with the other students in their lab group.
- When we began this study of chemical change, you all had some initial ideas about it and what it was all about. In just a few sentences, I want you to write what it is that you used to think about chemical changes. Take a minute to think back and then write down your response to “I used to think…”
- Now, I want you to think about how your ideas about chemical change have changed as a result of our discussion of the chemical changes within pizza dough. Again, in just a few sentences write down what you now think about chemical change. Start your sentences with, “Now, I think…”
To introduce the exploration activity the teacher will have students become more familiar with the concept of chemical reactions. To bring a familiar context to the idea of reactants and products, continue to build on the idea of cooking introduced at the beginning of the unit with the pizza video and activity.
- Show the video, “Healthy Eating - Cooking Across Generations”
- This video is the story of a young man named Jalani from St. Thomas in the Caribbean. He enters and wins a contest by creating a healthy recipe that includes important elements related to his cultural heritage and family traditions. Foods mentioned in the video are guava tart, tamarind stew, pea stew, and callaloo (or kallaloo). Jalani’s original recipe is Hurricane Lentil and Salmon Patties.
- Ask students to think of foods they eat at home that are special to their family or their cultural heritage.
- Just as in the example of Jalani, we may like these foods due to a special ingredient or seasoning.
- Tell students to think about their cultural and/or favorite foods. Use these questions to guide the class discussion.
- Are the ingredients easy to find?
- How is it prepared?
- What nutrients does the food provide?
- Tell the students you want to take a moment to look more closely at one of Jalani’s family favorite ingredients to include in their cooking. Show the class a video that explains the chemical reaction involved in cooking with onions. Show the video, “The Chemistry of Onions”
The Chemistry of Onions - Tell the students to listen, watch for, and write on their own paper these three things.
- Why is this an example of a chemical reaction?
- What are the starting materials (reactants)?
- What are the ending materials (products)?
- After reviewing with the students their answers to these three questions, give each student a copy of the Student hand out: Library for Self-Documentation Template Chem Reactions (attached). Note: This template may be adapted for use with every SOL objective. This is why it is called a library. By the end of the school year the student would have accumulated a personal library of science in her/his world.
- Explain to students this library will be their record of two chemical reactions that are important to them.
- Read the example written in Entry 1 describing Jalani’s original recipe.
- Read with the students the directions for entries 2 and 3.
- Read with the students the directions on page 2 of the hand-out. On page two the students will write the name of one of their two examples from page one as the topic to describe as they answer the questions on page two.
- The purpose of this activity is to allow students to find a personal connection to our academic content. Allowing students to find personal relevance for the academic content is known to enhance student comprehension and retention. “Culture guides how we process information.” (p. 48, Culturally Responsive Teaching and the Brain, by Zaretta Hammond). This activity taps into a student’s existing funds of knowledge to form connections that motivate a student to engage in the cognitive struggle of learning more about chemical reactions.
- The completion of the student’s entries will take time. On the day the assignment is due, plan time for a class discussion.
- One way of using this to build your class community could be to have students find another student in the room who has a similar reactant or product.
- Once the pairs form, ask the students to find another pair with a common factor.
- Then, ask the groups of four to discuss the question, “What factor about each person’s chemical reaction brought all of you together into the same group? In other words, what did you all have in common?”
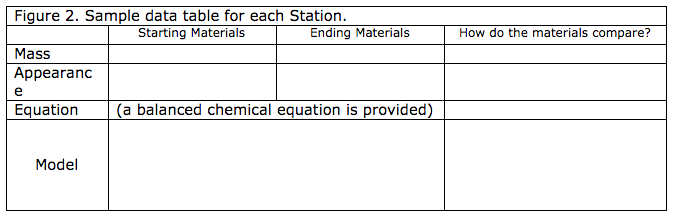
To continue to explore the topic of the Law of Conservation of Matter, students conduct three chemical change experiments and make models of the chemical reactions to investigate the Law of Conservation of Matter. This module has an accompanying student response paper with directions for each station. The student response paper has space for students to record the mass of the lab items before and after the chemical reaction (see Figure 2). It is important to discuss and follow lab safety with the students. Discuss with students proper lab measurement techniques. Student data should be the mass of the chemicals without the mass of the containers.
Teacher preparation for this investigation requires organization of materials to allow students to transition from completing one lab experiment to making a model of the chemical reaction. It is recommended to set up each station as a pair of containers. One container with the lab supplies, called the “Reaction Box”, and one container with the modeling materials, called the “Model” box.
The modeling materials need to be three-dimensional. For each chemical reaction provide a different color of modeling clay/dough or craft foam material (i.e. salt dough, craft foam mini geometry shapes) for each element in the chemical reaction. Students are provided the balanced chemical equation on their lab paper. The teacher should provide a key for the color scheme for each element. A printable model guide to put in the box is provided with this module. The guide for the model introduces students to chemical equation vocabulary. For example, the students will see the chemistry name for “Starting Materials” is reactants.
The teacher explains to the students they will have two parts to complete for each lab station. The template in Figure 2 shows the organization of the data for each Station.
- First, students complete the lab procedure using the materials in the “Reaction” box.
- The teacher sets up the “Reaction” box with all supplies needed to conduct the experiment for one chemical reaction.
- Students record the mass and appearance of the substances before the chemical reaction in the “starting materials” column.
- Students record the mass and appearance of the substances after the chemical reaction in the “ending materials” column.
- Students write a description comparing these properties of the materials before and after the experiment.
- Students clean up the lab materials and return the supplies for conducting the experiment back to the chemical reaction box.
- Second, students complete the modeling activity using materials in the “Model” box.
- The teacher sets up the “Model” box with modeling materials for one chemical reaction.
- Modeling materials may be homemade salt dough or craft foam. The supplies in each box should have a specific color code key to identify one color for one element.
- Students use the materials provided by the teacher to make a three-dimensional model of the balanced chemical equation.
- Students draw a picture of the model in the data table.
- Students clean up the modeling materials and return the supplies to the “Model” box.
- As a scaffold for students overloaded by the visual in the Exploration Guides for each model, the teacher could break down the steps for building the model into a series of numbered steps (similar to directions for assembling a Lego kit).
- Another way to scaffold is to provide students with a colored pencil to match the color of modeling dough to be used for each different element. Have students color the symbols on a paper copy of the guide. Then, build the model.
Station 1: Lead (II) nitrate combines with sodium iodide
Teacher prep per lab group: Prepare lead nitrate solution – Carefully weigh out 5 g of lead (II) nitrate. Dissolve the lead nitrate in 100 ml of water
Reaction Box 1 Materials: lead nitrate solution, sodium iodide, water, graduated cylinder, balance, beaker, weighing paper, chemical spatulas
Model Box 1 Materials: five different colors of modeling materials, model guide (printed copy)
Station 2: Sodium hydroxide combines with hydrochloric acid
Teacher prep: If you have concentrated solutions of chemicals, dilute to 0.5 M concentration
Reaction Box 2 Materials: 0.5 M sodium hydroxide solution, 0.5 M hydrochloric acid, bromothymol blue (indicator), dropper, graduated cylinders, beaker, balance.
Model 2 Box Materials: four different colors of modeling materials, model guide (printed copy)
Reaction 3
NaHCO3+ C6H8O7 🡪3CO2 +3H2O +C6H5Na3O7
Teacher prep:
The Alka Seltzer is a combination of NaHCO3 and C6H8O7 in a solid form. Therefore, the chemicals cannot react until put in water. Provide enough tablets in the Reaction supply box so there is one per lab group.
Show students how to keep hold on the tablet within the end of the plastic bag off to the side while securing the plastic bag around the outside of the rim of the beaker with a rubber band. It is important to trap all gas from the chemical reaction within the bag and beaker. This will allow students to have an accurate measurement of the total mass of the “Ending Materials” (products).
Reaction Box 3 Materials: Alka Seltzer tablets, water, plastic bags, rubber band, graduated cylinders, beaker, balance.
Model Box 3 Materials: four different colors of modeling materials, model guide (printed copy)
Lab Analysis Students complete a strategy called Claim – Evidence – Reasoning with their lab group to develop their own evidence-based answer to the driving question, “How is matter conserved during a chemical change?” The teacher should move among the groups as they work to discuss their thinking. Make notes of who has good insight and ideas that will be productive for the whole class discussion to follow. Also, guide students with misconceptions to take a second look at their lab evidence and put their thoughts in context of the examples they have seen.
Once each group has their Claim – Evidence – Reasoning chart prepared, use these as the basis of a class discussion to put all ideas together into one class discovery chart to define the Law of Conservation of Matter.
Explanation
The teacher begins the next lesson by referring to the class discovery chart about the Law of Conservation of Matter compiled at the conclusion of the previous activity. The teacher will show and discuss with students video clips about the Law of Conservation of Matter and balancing chemical equations. Class discussion should make comparisons between the content of this direct discussion with the students’ claim-evidence-reasoning charts.
The class discussion may be affirmed with the next two video clips. Students watch, discuss, and take notes on these video clips. While watching the first video, the teacher introduces and models with the video demonstration the meaning of a closed system.
Teacher instruction on a closed system
- Student notes section B. This demonstration of the chemical reaction with Na2SO4 (sodium sulfate) and CaCl2 (calcium chloride) is done in a closed system.
- As you study science you will find it helps you to reason effectively and figure things out when you look at a situation as a _system__
- We call this experiment a __closed___ system.
- This is a closed system because the container (flask with rubber stopper) provides a boundary to separate what is inside the container from the _environment_ around it.
- In a closed system the only thing that may enter or leave is_energy_. In our example, thermal energy may enter and leave through the glass walls of the flask by conduction or radiation. However, the amount of matter did not change.
Following the list of additional sample videos you may wish to use, you find a series of equations recommended to be done with the students as guided practice. These practice questions are included on a student hand out included with this module. Then, have the students write a brief summary of the three things important things when balancing a chemical equation: (1) What must I count? (2) What must I compare? (3) What must I decide?
Sample videos
If your school has a subscription with brainpop.com there are two good videos. One is called, “Chemical Equations – Get the Balance Right”. The other is called, “The Law of Conservation”.
Go to Professor Dave's video about how to balance chemical equations
Attached: Guided Practice for Balancing Chemical Equations
After the guided practice, students are ready to practice independently. Three options are provided. It is an important practice for the teacher to move among the students and sit with different students to aske a specific question about her/his thinking while completing the interactive practice with a computer simulation.
A second option is to have students take turns while working in pairs. For example, while student one is operating the keyboard, student 2 is telling student one what to enter. Every five minutes the students switch roles. This is an opportunity for students to talk about the process of balancing chemical equations and develop their computational thinking skills.
Sample websites for practice balancing chemical equations
Elaboration
The conclusion of this module provides students an opportunity to apply their understanding of the Law of Conservation of Matter and practice their science process skills. Assign students to work with a partner.
Student Scenario You have been studying the Law of Conservation of Matter in Science class. Two members of your lab group have missed class. You and your partner are to write a lab procedure that demonstrates the Law of Conservation of Matter so the absent students may follow when they return to school.
You want to make sure your friends will really understand what they are doing.
- write a procedure
- test the procedure
- collect data
- write a meaningful explanation
The students must be able to follow your procedure to conduct an investigation with vinegar and baking soda to gain enough evidence to write their own Claim – Evidence – Reasoning chart to prove matter is conserved during a chemical change.
You must follow all safety rules and use the materials provided by your teacher.
Teacher Tips
Questions the teacher may ask students as they are planning their project.
What do you think will happen when vinegar is added to baking soda?
What is your idea for organizing the materials?
What do you already know about this that makes you think so?
Can you state your prediction to show what you think will happen or change? (When I do this ___________, I think that __________will be the result.)
Questions the teacher may ask students as they write the procedure.
What steps will you follow to test your prediction? Write each step as you do it.
What materials will you need?
What are the variables?
Write out each step so someone else could do it from your directions.
What will you need? Try to be specific. Do not forget your tools for measuring.
What are the factors that will stay the same? What might change?
What will you observe?
How will your classmates record evidence that will help them prove the law of conservation of matter?
Evaluation
Module Assessment Attached
Module Review
This module allows students experiential evidence of the Law of Conservation of Matter. Students engage in modeling chemical reactions which they have conducted in the lab. This connection takes students from their macroscopic experiences to a submicroscopic model.
Additional Resources
For more information about Virginia Commonwealth University's School of Education STEM initiatives, visit the Center for Innovation in STEM Education (CISTEME).