
https://goopenva.org/editor/documents/376
- Subject:
- Computer Science
- Mathematics
- Science
- Material Type:
- Teaching/Learning Strategy
- Author:
- Kelly Bucceri
- Date Added:
- 07/29/2023

https://goopenva.org/editor/documents/376

Before Ernest Rutherford's famous gold foil experiment in 1911, it was not known how the positive part of the atom was distributed. His experiment showed that if you shot positively charged particles at the atoms in a very thin sheet of gold foil, that very rarely, a particle would bounce back from the foil rather than going straight through it. Experiment with changing the distribution of positive charge and see how it affects the paths of positively charged particles moving near it.

This interactive, scaffolded activity allows students to build an atom within the framework of a newer orbital model. It opens with an explanation of why the Bohr model is incorrect and provides an analogy for understanding orbitals that is simple enough for grades 8-9. As the activity progresses, students build atoms and ions by adding or removing protons, electrons, and neutrons. As changes are made, the model displays the atomic number, net charge, and isotope symbol. Try the "Add an Electron" page to build electrons around a boron nucleus and see how electrons align from lower-to-higher energy. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology. The Concord Consortium develops deeply digital learning innovations for science, mathematics, and engineering. The models are all freely accessible. Users may register for additional free access to capture data and store student work products.

This interactive activity helps learners visualize the role of electrons in the formation of ionic and covalent chemical bonds. Students explore different types of chemical bonds by first viewing a single hydrogen atom in an electric field model. Next, students use sliders to change the electronegativity between two atoms -- a model to help them understand why some atoms are attracted. Finally, students experiment in making their own models: non-polar covalent, polar covalent, and ionic bonds. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology.
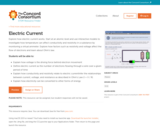
This 90-minute activity features six interactive molecular models to explore the relationships among voltage, current, and resistance. Students start at the atomic level to explore how voltage and resistance affect the flow of electrons. Next, they use a model to investigate how temperature can affect conductivity and resistivity. Finally, they explore how electricity can be converted to other forms of energy. The activity was developed for introductory physics courses, but the first half could be appropriate for physical science and Physics First. The formula for Ohm's Law is introduced, but calculations are not required. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology. The Concord Consortium develops deeply digital learning innovations for science, mathematics, and engineering.
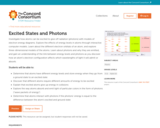
This concept-building activity contains a set of sequenced simulations for investigating how atoms can be excited to give off radiation (photons). Students explore 3-dimensional models to learn about the nature of photons as "wave packets" of light, how photons are emitted, and the connection between an atom's electron configuration and how it absorbs light. Registered users are able to use free data capture tools to take snapshots, drag thumbnails, and submit responses. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology.
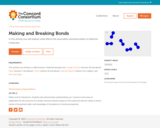
In this interactive activity, learners explore factors that cause atoms to form (or break) bonds with each other. The first simulation depicts a box containing 12 identical atoms. Using a slider to add heat, students can see the influence of temperature on formation of diatomic bonds. Simulations #2 and #3 introduce learners to reactions involving two types of atoms. Which atom forms a diatomic molecule more easily, and why? The activity concludes as students explore paired atoms (molecules). In this simulation they compare the amount of energy needed to break the molecular bonds to the energy needed to form the bonds. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology.
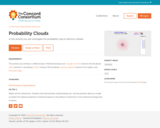
In this interactive activity, learners build computer models of atoms by adding or removing electrons, protons, and neutrons. It presents the orbital model of an atom: a nucleus consisting of protons and neutrons with electrons surrounding it in regions of high probability called orbitals. Guided tasks are provided, such as constructing a lithium atom and a carbon-12 atom in the fewest possible steps. The activity concludes with a model for building a charged hydrogen atom (an ion). Within each task, students take snapshots of their work product and answer probative questions. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology.
Elementary grade students investigate heat transfer in this activity to design and build a solar oven, then test its effectiveness using a temperature sensor. It blends the hands-on activity with digital graphing tools that allow kids to easily plot and share their data. Included in the package are illustrated procedures and extension activities. Note Requirements: This lesson requires a "VernierGo" temperature sensing device, available for ~ $40. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology. The Consortium develops digital learning innovations for science, mathematics, and engineering.

This model shows the combined effects of heat capacity and conductivity.

The heat conductivity of a solid material defines how fast heat will flow through it. You can probably think of several everyday examples of materials with high (fast) conductivity or low (slow) conductivity. This model illustrates the effect of different conductivities by placing different materials between a hot and a cold object and graphing the changing temperatures.
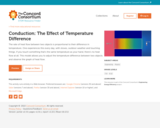
The rate of heat flow between two objects is proportional to their difference in temperature. One experiences this every day, with stoves, outdoor weather and touching things. If you touch something that's the same temperature as your hand, there's no heat flow at all. This model allows you to adjust the temperature difference between two objects and observe the graph of heat flow.
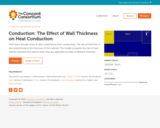
Heat flows through solids at rates measured by their conductivity. The rate of heat flow is also proportional to the thickness of the material. This model compares the rate of heat transfer between two objects when they are separated by walls of different thickness.

Experiment with conductivity in metals, plastics and photoconductors. See why metals conduct and plastics don't, and why some materials conduct only when you shine a flashlight on them.

Explore how populations change over time in a NetLogo model of sheep and grass. Experiment with the initial number of sheep, the sheep birthrate, the amount of energy sheep gain from the grass, and the rate at which the grass re-grows. Remove sheep that have a particular trait (better teeth) from the population, then watch what happens to the sheep teeth trait in the population as a whole. Consider conflicting selection pressures to make predictions about other instances of natural selection.

Science Instructional Plans (SIPs) help teachers align instruction with the Science Standards of Learning (SOL) by providing examples of how the content and the scientific and engineering practices found in the SOL and curriculum framework can be presented to students in the classroom.
In this physical science lab, students investigate whether or not chewing gum should be considered eating. Students plan their own experiments for this lab. They use the law of conservation of mass to reason that the portion lost of the original mass of gum must be swallowed. Students determine the portion of original mass of gum. A student lab sheet and CER template are provided.

The lessons in this module are empirical – abductive. The teacher helps students identify the activity of substances within pizza dough. The teacher announces the students will conduct chemical reactions to explore how matter is conserved during a chemical change. After the class compares their reasoning, the teacher provides clarifying and direct instruction with videos, guided practice and supported computer simulation practice for students to learn to balance chemical equations. Students complete a problem-based investigation to apply their learning by writing, testing and explaining a lab procedure that will help an absent classmate to gather evidence and gain an understanding of the Law of Conservation of Matter. This module was developed by Patricia Kramolisch as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.

Water is a natural resource that is limited and needs to be conserved. Students will collect data about how much water they are using at home, design a plan to conserve water, implement the plan, and again take data to determine their success.

Description: Water is a natural resource that is limited and needs to be conserved. Students will collect data about how much water they are using at home, design a plan to conserve water, implement the plan, and again take data to determine their success.