
Explore the potential and kinetic energy as two atoms form a covalent bond.
- Subject:
- Science
- Material Type:
- Simulation
- Provider:
- Concord Consortium
- Provider Set:
- Concord Consortium
- Author:
- Concord Consortium
- Date Added:
- 06/28/2022

Explore the potential and kinetic energy as two atoms form a covalent bond.
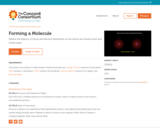
Explore the balance of forces and electron distribution as two atoms are moved closer and further apart.
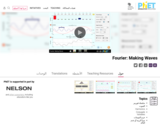
Learn how to make waves of all different shapes by adding up sines or cosines. Make waves in space and time and measure their wavelengths and periods. See how changing the amplitudes of different harmonics changes the waves. Compare different mathematical expressions for your waves.

VDOE resource to assist in the tracking of content in fourth grade science. This is a resource that divisions or schools may use as needed.

After experiencing this module, students should understand that friction is a force that opposes motion (SOL 5.3e). This lesson was written with an inclusive fifth grade classroom in mind, but can be adapted for a smaller pull-out group if necessary. This module was developed by Karin Kaerweras part of a Virginia Commonwealth University STEM initiative sponsered by the Virginia Department of Education.
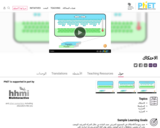
Learn how friction causes a material to heat up and melt. Rub two objects together and they heat up. When one reaches the melting temperature, particles break free as the material melts away. Arabic Language.

Science Instructional Plans (SIPs) help teachers align instruction with the Science Standards of Learning (SOL) by providing examples of how the content and the scientific and engineering practices found in the SOL and curriculum framework can be presented to students in the classroom.

In this lesson, students will experience the force of friction as they use block-based coding to program a Sphero Bot through a maze made up of materials with varying amounts of friction.

To combat the common misconception that all mutations have large effects on proteins, students experiment with the Protein Synthesis Simulation to learn about the relationship among DNA, codons, amino acids, and proteins. At first, students investigate a strand of DNA that includes all 20 amino acids. Then, they make guided changes to discover that sometimes a single change can stop most of the protein from being formed, while another change produces no noticeable affect at all. Next, they complete challenges to mutate a DNA strand, and conclude with a mini-research project on mutations.
In this hands-on activity students learn how a gene provides the instructions for making a protein, and how genes can cause albinism or sickle cell anemia. Simple paper models are used to simulate the molecular processes of transcription and translation. This activity can be used to introduce students to these topics or to reinforce student understanding. In addition, students evaluate the advantages and disadvantages of different types of models included in this activity.

Rocks can be classified based on their formation. This formation process takes time but is a circular process that allows rocks to interchange forms between metamorphic, igneous, and sedimentary rocks. This lesson will allow students to “create codes” that allow a rock to experience the proper changes to change forms.
Objectives:
- To help students learn which food is fruit.
- To introduce the concept of healthy food.
- To introduce concept of where food comes from.
In this MS/HS unit supported by NASA, students engage with online interactives, authentic datasets, and citizen science protocols to construct models and explanations for the unit driving question, "How do landscapes recover after a wildfire?"
In this activity, students study gas laws at a molecular level. They vary the volume of a container at constant temperature to see how pressure changes (Boyle's Law), change the temperature of a container at constant pressure to see how the volume changes with temperature (Charles’s Law), and experiment with heating a gas in a closed container to discover how pressure changes with temperature (Gay Lussac's Law). They also discover the relationship between the number of gas molecules and gas volume (Avogadro's Law). Finally, students use their knowledge of gas laws to model a heated soda can collapsing as it is plunged into ice water.
This activity addresses Boyle's Law from a physiological perspective.
This activity uses the phenomena of hydrogen-filled weather balloons and hot air balloons to explore some of the gas laws.

Explore how a particle model of gases works to predict the behavior of a syringe under various conditions.
Pump gas molecules to a box and see what happens as you change the volume, add or remove heat, change gravity, and more. Measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other.

Science Instructional Plans (SIPs) help teachers align instruction with the Science Standards of Learning (SOL) by providing examples of how the content and the scientific and engineering practices found in the SOL and curriculum framework can be presented to students in the classroom.

The goal of this exercise is to explore gender role attitudes in Japan and how they vary across different parts of the population. Crosstabulation will be used.