
Students will participate in multiple hands on activities with states of matter over several days.
- Subject:
- Data and Analysis
- Matter
- Material Type:
- Activity/Lab
- Interactive
- Author:
- Katherine “Faith” Bailey
- Date Added:
- 04/29/2024

Students will participate in multiple hands on activities with states of matter over several days.

Science Instructional Plans (SIPs) help teachers align instruction with the Science Standards of Learning (SOL) by providing examples of how the content and the scientific and engineering practices found in the SOL and curriculum framework can be presented to students in the classroom.
The students will analyze the pH of four citrus fruits: lime, lemon, orange, and a grapefruit, and then they will analyze the pH of four different types of stain removers:
Shout Advanced, Spray 'n Wash Max, Oxi Clean Laundry, and Clorox Oxi Magic. Using their learned information, they will predict and then analyze the pH of a final cleaning solution Bathroom Lime & Scale Remover, which is a Green product.
Students will use two methods of determining the pH for comparison. This will be done utilizing pH litmus paper and creating a cabbage indicator solution.
Students will also read labels, list the first few ingredients, and identify sales tactics used for their products such as eye-catching phrases. (Language Arts)
Finally, students will discuss and share their results as a class. Students need to answer what do citrus fruits have to do with cleaning? What product is most effective? Students need to support their answer with data from their experiment.

This science instructional plan (SIP)
Explore the interactions between various combinations of two atoms. Turn on the force arrows to see either the total force acting on the atoms or the individual attractive and repulsive forces. Try the "Adjustable Attraction" atom to see how changing the parameters affects the interaction.
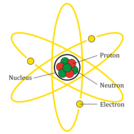
This resource is designed to accompany students notes, texts, and other instructional resources and provide a means to review what they have learned about atomic structure and counting atoms in compounds.

I love this activity, and I think it is a great instructional resource for students to learn about atomic structure and counting atoms in compounds. However, I would like to add a single modification.MODIFICATION: Add the following question: How are atoms of one element different from the atoms of a different element? This resource is designed to accompany students notes, texts, and other instructional resources and provide a means to review what they have learned about atomic structure and counting atoms in compounds.
5th grade students that are studying matter will be focusing in this lesson on the smallest unit of matter - the atom.
Students will use small marshmallows to construct a model of an atom.

This is a Biochemistry Pre-test or Review, matching exercise. In the form of a one page Word document. It is the first unit in AP Biology with these terms being used throughout the rest of the year, so it is essential learning for that course.

The foamy fun of "Elephant's Toothpaste," also known as the catalytic decomposition of hydrogen peroxide, helped Camille Schrier win her job as Miss America 2020! In this episode, Camille re-creates this winning chemical reaction and teaches us all about the science of catalysts and decomposition. Explore questions such as: What is a catalyst? What does a catalyst do? Why do we need a catalyst to make "Elephant's Toothpaste"? It’s a HUGE, wonderful, foamy mess that's all powered by science! Developed for students in grades 6 - 10.
Primary Strand
SCI.5.7.c Explain the role of energy in changing the phase of matter of a substance.
Integrated Strand
Reading 5.5a Summarize events in the plot in a fiction text
Reading 5.5m Use reading strategy of visualizing when reading fiction and nonfiction
In this lesson, students will read the mentor text What's the Matter With the Three Little Pigs? Students will summarize at stopping points throughout the book. Students will also find three examples from the book and how the matter changed.
This activity covers balancing chemical equations, endothermic/exothermic reactions, and identifying the reactants and products. I used this activity as a homework assignment to reinforce what had been taught in class that day. It could easily be adapted for use as a warm-up, extension activity, extra credit, reinforcement, etc.

After the completion of this module students will understand that sound travels in compression waves and must have a medium to travel. Sound also travels in liquids and gases. Students will also understand that sound waves are created by vibrations and capable of transmitting energy.This module was developed by Sarah Donnelly as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.

These are simple ways to demonstrate water chemistry concepts such as adhesion, cohesion, and polarity to students in a hands on way using easy to find and inexpensive lab materials. The lesson was originally designed to align with a biology course to teach the impact of water on life, but it could also be modified to fit a chemistry or physical science curriculum.

This module focuses on SOL 5.7c, energy’s effects on phases of matter. The target population is a fifth grade inclusive classroom. After this learning experience, students should understand that as its temperature increases, many kinds of matter change from a solid to a liquid to a gas. As its temperature decreases, that matter changes from a gas to a liquid to a solid. This module was developed by Karin Kaerwer, Stephanie Hooks, Sarah Donnelly as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.
As technology has evolved over time so has the understanding of the structure of the atom. This module focuses on how the model of the atom has changed over time using The Atomic Theory Timeline including the historical contributions of the scientists involved. This module was developed by Tracey Nipper as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.
Students hang an object of known mass from a spring scale and submerge it to different depths in a liquid. By analyzing the change in the upward force of the spring scale, students are able to determine the density of the liquid.

Do you think we can blow up a balloon using only ingredients from the pantry? Using simple, safe, at-home materials, we will explore the concepts of pH and acid-base chemistry and have some fizzy fun! With their signature gas-producing fizz, the acid-base reactions in this episode are both fun and functional. Not only will the reaction blow up a balloon, it also makes your bath bomb fizz in the tub. Join Miss America 2020 to cook up some science in your kitchen, and learn more about the chemistry of fizzy fun! Developed for students in grades 6- 10.
Students will explain that matter consists of atoms held together by electromagnetic forces and exists as different substances which can be utilized based on their properties. Students will be able to describe the behavior of atoms during a chemical change. Students will be able to distinguish covalent and ionic bonds.This module was developed by Patricia Kramolisch as part of a Virginia Commonwealth University STEM initiative sponsored by the Virginia Department of Education.

Hands on Chemistry is a resource designed for use in traditional, online, and blended high school Chemistry classrooms. It was developed by Blue Ridge PBS in collaboration with Virtual Virginia. In this video, Chemistry teacher Fred Mitchell demonstrates the process of synthesizing nylon.
Polymer, forceps, nylon.
VA SOL CH.6